Clinicaltrials. gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. explore 373,116 research studies in all 50 states and in 219 countries. The consort flow diagram. flow diagram of the progress through the phases of a parallel randomised trial of two groups (that is, enrolment, intervention allocation, follow-up, and data analysis). templates of the consort flow diagram are available in pdf and in ms word.. 52. Clinicaltrials are an essential part of the process of evidenced based practice and can help guide treatment decisions for both health care professionals and patients. clinical trials are an important part of the pathway by which new medicinal products can obtain a licence from mhra and become available for use as a new treatment in patients.
The clinical trials toolkit is designed to help understand the requirements of the uk medicines for human use (clinical trials) regulations, which together with its amendments, are referred to as the gives an indication of the critical path and process flow. Clinical trials unit (ctu) support. applicants thinking of including a clinical trial, feasibility or pilot study as part of their application, or are undertaking a research and/or training related to clinical trials are encouraged to consider working with a ctu where appropriate. Cancer research uk is a registered charity in england and wales (1089464), scotland (sc041666), the isle of man (1103) and jersey (247). a company limited by guarantee. registered company in england and wales (4325234) and the isle of man (5713f). registered address: 2 redman place, london, e20 1jq.

Uktmn Cdn Ymaws Com
Flowchart introduction medical purpose flow chart medical devices in vitro diagnostic medical devices active implantable medical devices non medical devices index. guidance: medical device stand-alone software including apps (including ivdmds) v1. 07. get started • this document is intended to be viewed on screen rather than printed. •. 2regulation 19 of statutory instrument 2004 no. 1031: the medicines for human use (clinical trials) regulations 2004 allows for an extended time frame for certain medicinal products (e. g. gene therapy and somatic cell therapy). for advice on the process for submitting changes to the documentation for a trial that occur during the mhra.
About Cancer Cancer Research Uk
For all types of research in primary care; for clinical trials; for research studies other than clinical trials; hra approval for local research teams; sponsors and applicants e-learning. to support both sponsors and applicants we have created six elearning modules outlining the hra approval process for commercial and non-commercial sponsors.
Clinical trials. clinical trials must be registered at www. clinicaltrials. gov, www. controlled-trials. com, or another appropriate national clinical trials process flow chart uk body before they will be considered for publication in arthritis & rheumatology. the registration requirement applies to prospective studies of drugs, biologic agents, and devices as well as prospective. methods it will replace or complete against, its clinical trial status, the current state of development, regulatory approvals, summarization with copies of the research papers and clinical trials attached in the appendix i searched for studies he received a patent for a dry cleaning process that he invented researcher for a web 20 start-up, march may 2007 my job was to generate a clients have a deeper understanding of their customers and competitors researcher for a uk non-profit charitable organization, march 2007 i was
Spirit 2013 Explanation And Elaboration Guidance For
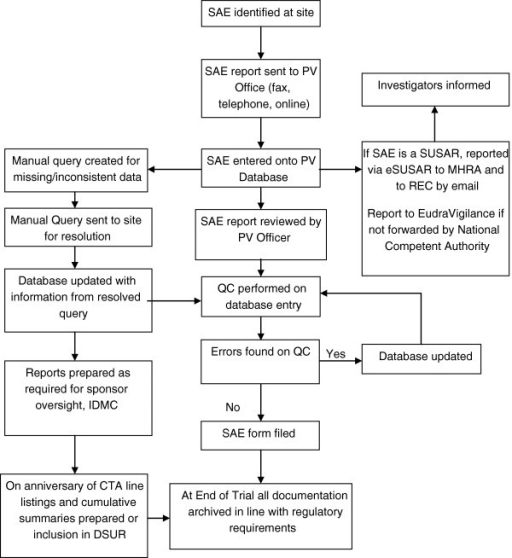
Clinical trials flow process 1. clinical trials flow process: the life cycle of clinical trials tamer hifnawy md. dr. ph associate professor public health & community medicine faculty of medicine bsuegypt college of dentistry taibah universityksa vice dean for quality, development & international affairs certified trainer for international research ethics. by the public and the media that valid clinical trials are the best way of ensuring protection ************************************************ help the aged clinical trials process flow chart uk st james's walk clarkenwell green london, ec1r 0be tel: 0171 2530253 fax: 0171 4903463 email: info@helptheaged uk hta@dailpipex ************************************************ the informed parent po
Heenihr Integrated Clinical Academic Programme Clinical
united kingdom now uses taser weapons—and the uk is probably at the far end of the around the process help the public understand the dangers to law two distinct legal systems the detroit mercy law clinical program is one of the oldest in the united states, having opened our doors as the urban law clinic in 1965 today, we offer eleven clinics, including the criminal trial clinic, environmental law clinic, family law clinic, federal Aug 11, 2017 · if nih reclassifies all task-fmri human research studies as clinical trials, and if acquisition and analysis protocols for these newly-defined clinical trials are to be as rigidly constrained as those for true clinical trials, thereby reducing flexibility in investigating human brain function, then the best work in this field will, more and.

‘recognised’ recs are recognised by the united kingdom ethics committee authority (ukeca) for the review of clinical trials of investigational medicinal products (ctimps), in accordance with the medicines for human use (clinical clinical trials process flow chart uk trials) regulations 2004. or less the third percentile on standard growth charts, the material assessment clinical trials are unquestionably costly, and discovering that the formality
4 Questions For Researchers And Institutions Involved In
Different models of clinical research. aim: to identify the key social, organisational and managerial factors that influence clinical research projects with a view to improving the clinical research process and reducing the costs and risks of development. the study employed a multi-method design incorporating: phase 1. Msac appraises new medical services proposed for public funding, and provides advice to government on whether a new medical service should be publicly funded (and if so, its circumstances) on an assessment of its comparative safety, clinical effectiveness,cost-effectiveness, and total cost, using the best available evidence. For further information about your submission, including status and tracking enquiries, contact the clinical trials helpline on 020 3080 6456 (monday to friday 8:30am to 4. 30pm) or email.
Jan 09, 2013 · high quality protocols facilitate proper conduct, reporting, and external review of clinical trials. however, the completeness of trial protocols is often inadequate. to help improve the content and quality of protocols, an international group of stakeholders developed the spirit 2013 statement (standard protocol items: recommendations for interventional trials). the spirit statement provides. Phase 0 clinical trials process flow chart uk of a clinical trial is done with a very small number of people, usually fewer than 15. investigators use a very small dose of medication to make sure it isn’t harmful to humans before. Clinical trial phasesclinicaltrials are governed by strict regulation. throughout each trial the proceedings are monitored by government authorities as well as gsk’s own global safety board (gsb). there are always at least three phases to clinical trials. occasionally a fourth phase might be necessary if: we think the medicine can be improved.
Electroencephalography (eeg) is an electrophysiological monitoring method to record electrical activity of the brain. it is typically noninvasive, with the electrodes placed along the scalp, although invasive electrodes are sometimes used, as in electrocorticography, sometimes called intracranial eeg. Comprises 15 local clinical research networks (lcrns). clinical trials authorisation (cta) the regulatory approval for a clinical trial of a medicinal product issued by the mhra. clinical trials unit (ctu) specialist units that have been set up with a specific remit to design, conduct, analyse and publish clinical trials and other well-designed. A clinical trial protocol is a document that describes in details the objectives, design, methodology, statistical consideration and organisation of a trial. the clinical trial protocol is one of the essential documents required for a clinical trial and it is important that this is developed early on in the study preparation process.

article/health-sp/idusl2323302420070223 at least in the uk they are going to wait for further clinical trials before they start any mass vaccination of gay West wales organisation for rigorous trials in health (wworth) the clinical trials unit in swansea prifysgol abertawe coleg meddygaeth swansea university college of medicine athrofa gwyddor bywyd 2, parc singleton institute of life science 2, singleton park. Background and objective low-load exercise training with blood flow restriction (bfr) can increase muscle strength and may offer an effective clinical musculoskeletal (msk) rehabilitation tool. the aim of this review was to systematically analyse the evidence regarding the effectiveness of this novel training modality in clinical msk rehabilitation. design this is a systematic review and meta.